Syntaxin
| Syntaxin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Structure of an evolutionarily conserved N-terminal domain of syntaxin 1A.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Syntaxin | ||||||||
| Pfam | PF00804 | ||||||||
| InterPro | IPR006011 | ||||||||
| SMART | SM00503 | ||||||||
| SCOP | 1br0 | ||||||||
| OPM family | 218 | ||||||||
| OPM protein | 3hd7 | ||||||||
|
|||||||||
Syntaxins are a family of membrane integrated Q-SNARE proteins participating in exocytosis.[2]
Contents |
Domains
Syntaxins possess a single C-terminal transmembrane domain, a SNARE domain (known as H3), and an N-terminal regulatory domain (Habc). Syntaxin 17 may have two transmembrane domains.
- The SNARE (H3) domain binds to both synaptobrevin and SNAP-25 forming the core SNARE complex. Formation of this incredibly stable SNARE core complex is believed to generate the free energy required to initiate fusion between the vesicle membrane and plasma membrane.
- The N-terminal Habc domain is formed by 3
 -helices and when collapsed onto its own H3 helix forms an inactive "closed" syntaxin conformation. This closed conformation of syntaxin is believed to be stabilized by binding of Munc-18 (nSec1), although more recent data suggests that nSec1 may bind to other conformations of syntaxin, as well. The "open" syntaxin conformation is the conformation that is competent to form into SNARE core complexes.
-helices and when collapsed onto its own H3 helix forms an inactive "closed" syntaxin conformation. This closed conformation of syntaxin is believed to be stabilized by binding of Munc-18 (nSec1), although more recent data suggests that nSec1 may bind to other conformations of syntaxin, as well. The "open" syntaxin conformation is the conformation that is competent to form into SNARE core complexes.
Function
In vitro syntaxin per se is sufficient to drive spontaneous calcium independent fusion of synaptic vesicles containing v-SNAREs.[3]
More recent and somewhat controversial amperometric data suggest that the transmembrane domain of Syntaxin1A may form part of the fusion pore of exocytosis.[4]
Binding
Syntaxins bind synaptotagmin in a calcium-dependent fashion and interact with voltage dependent calcium and potassium channels via the C-terminal H3 domain. Direct syntaxin-channel interaction is a suitable molecular mechanism for proximity between the fusion machinery and the gates of 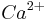 entry during depolarization of the presynaptic axonal boutons.
entry during depolarization of the presynaptic axonal boutons.
The Sec1/Munc18 protein family is known to bind to Syntaxin and regulate Syntaxins machinery. Munc18-1 binds to Syntaxin 1A via two distinct sites referred as N-terminus binding and "closed" conformation that incorporates both the central Habc domain and the SNARE core domain. Binding to N-terminus of Syntaxin-1 is thought to facilitate Syntaxin-1 interact with another SNARE. and Binding to "closed" conformation Syntaxin-1 is believed to be inhibitory regulate Syntaxin-1's function.
Recently published data show that alternative spliced Syntaxin 1 (STX1B) which lacks the transmembrane domain localizes in the nuclei.[5]
Genes
Human genes encoding syntaxin proteins include:
- STX1A, STX1B, STX2, STX3, STX4, STX5, STX6, STX7, STX8, STX10, STX11, STX12, STX16, STX17, STX18, STX19
References and notes
- ^ Fernandez I, Ubach J, Dulubova I, Zhang X, Südhof TC, Rizo J (September 1998). "Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A". Cell 94 (6): 841–9. doi:10.1016/S0092-8674(00)81742-0. PMID 9753330.
- ^ Bennett MK, García-Arrarás JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH (September 1993). "The syntaxin family of vesicular transport receptors". Cell 74 (5): 863–73. doi:10.1016/0092-8674(93)90466-4. PMID 7690687.
- ^ Woodbury DJ, Rognlien K (2000). "The t-SNARE syntaxin is sufficient for spontaneous fusion of synaptic vesicles to planar membranes". Cell Biol. Int. 24 (11): 809–18. doi:10.1006/cbir.2000.0631. PMID 11067766.
- ^ Han X, Wang CT, Bai J, Chapman ER, Jackson MB (April 2004). "Transmembrane segments of syntaxin line the fusion pore of Ca2+-triggered exocytosis". Science 304 (5668): 289–92. doi:10.1126/science.1095801. PMID 15016962.
- ^ Pereira S, Massacrier A, Roll P, Vérine A, Etienne-Grimaldi MC, Poitelon Y, Robaglia-Schlupp A, Jamali S, Roeckel-Trevisiol N, Royer B, Pontarotti P, Lévêque C, Seagar M, Lévy N, Cau P, Szepetowski P (November 2008). "Nuclear localization of a novel human syntaxin 1B isoform". Gene 423 (2): 160–71. doi:10.1016/j.gene.2008.07.010. PMID 18691641.
External links
|
||||||||||||||||||||||||||||||||||||||||||||